治療とイメージングは経直腸プローブ内の同一トランスデューサで行い、治療部位が正確に把握できるだけではなく治療中のモニタリングがリアルタイムで可能なので、安全な治療が出来ます。(焦点領域のみを高温(80~100℃)で前立腺を切除、患者のQOL、医療機関のNEEDSを両立させる低侵襲、低コスト治療を実現)。
このサイトは、弊社が販売する製品に関する情報を、医療関係者の方に提供することを目的として作成されています。
一般の方への情報提供を目的としたものではありませんのでご了承ください。
あなたは医療従事者ですか?

ソナブレート500
超音波手術器
20800BZG00025000
治療とイメージングは経直腸プローブ内の同一トランスデューサで行い、治療部位が正確に把握できるだけではなく治療中のモニタリングがリアルタイムで可能なので、安全な治療が出来ます。(焦点領域のみを高温(80~100℃)で前立腺を切除、患者のQOL、医療機関のNEEDSを両立させる低侵襲、低コスト治療を実現)。

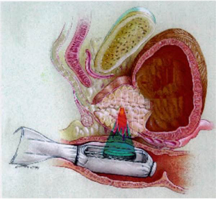
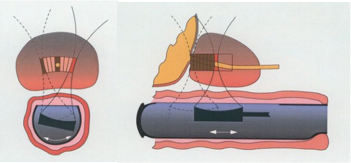
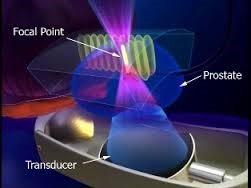
原理:治療はトランスデューサから高エネルギー超音波を照射し狭い焦点で超音波を集束させ、焦点領域の温度を数秒で80~100℃に上げて、組織を凝固壊死させます。(下図)
強力超音波を円形凹面振動子(プローブ)から照射すると、その振動エネルギーは曲率中心に収束します。
焦点領域の温度を80~100℃に上昇させます。HIFUはこの原理を利用して治療部位を凝固壊死させます。しかも焦点領域以外の、周辺あるいは介在組織へは影響をほとんど与えません。
治療域:高エネルギー超音波を前立腺の一点に集めることにより、その部分のみを約90℃の高温で治療します。
下記の図にあるように直腸から挿入されたプローブより照射される強力超音波は、膀胱頸部から精丘にかけての前立腺部尿道の選択した範囲のみを照射します。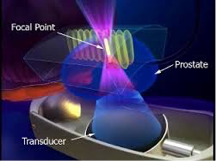
上記のHIFU治療の原理は、例えて言うと、太陽の光をレンズで集めて一点だけを高温にするようなものです。高エネルギーの超音波を発生させ、それを体内の一点に集めるのです。そうすると、その点の温度が90℃くらいまでになります。超音波が集束された部分だけが高温になり、他の組織にはダメージはほとんどありません。
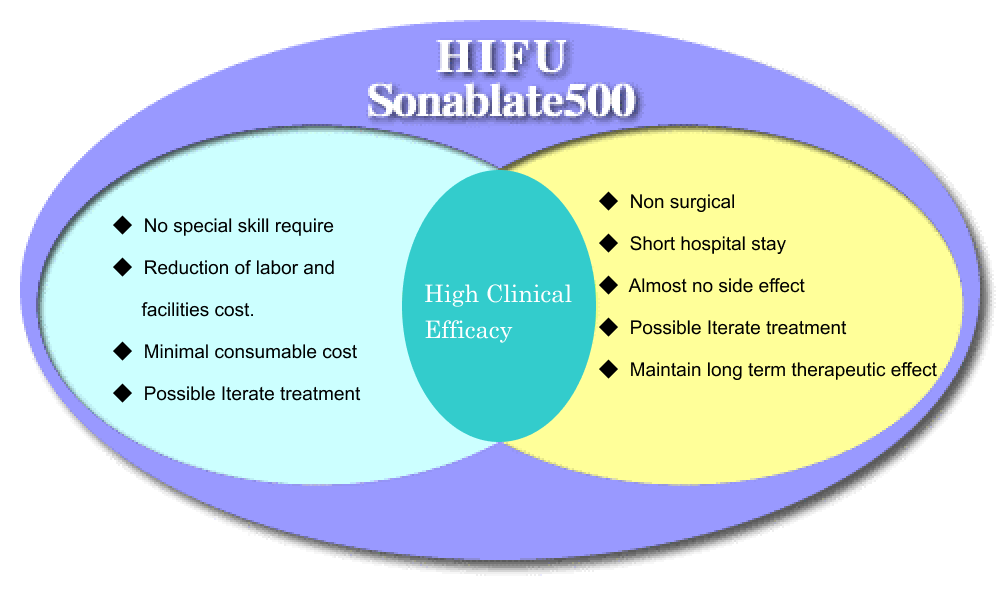
この治療法は患者さんの肉体的負担が少ないだけでなく、入院期間の短かさや必要な消耗品の少なさから治療コストを抑えることが可能です。Sonablate500は患者さんのQOLと医療機関のNEEDSを両立させることができます
| 文献名 | 著者 | アブストラクト |
| Focal Targeted Therapy Will Be a Future Treatment Modality for Early Stage Prostate Cancer | Jean J.M.C.H. de la Rosette, Vladimir Mouraviev, Thomas J. Polascik * |
Context: Focal targeted therapy of early stage prostate cancer (PCa) can ideally facilitate
the concept of personalized medicine in contemporary surgical oncology. Objective: To present indications and outcomes of subtotal glandular ablation. This treatment approach aims at the elimination of the cancer with preservation of uninvolved tissue in an attempt to maintain a patient’s quality of life (QoL), including undisturbed erectile function as well as urinary and bowel control. Evidence acquisition: In 2002, the idea of a ‘‘lumpectomy’’ using an organ-sparing approach for very localized PCa was proposed in parallel with organ-sparing breast cancer treatment in women. Since then, a few pilot clinical studies have demonstrated an acceptable short-term cancer control while minimizing the complication rate. At the same time, progress in PCa screening has led to a significant stage and tumor volumemigration toward early stage disease. In the past fewyears, a collection of accumulated data has created a scientific background for further development of this concept toward a wider implementation into clinical practice. In this paper, we review all available literature from PubMed of the past 15 yr—from 1994 to 2008— including the terms localized prostate cancer, focal therapy, organ preservation, and morbidity. Evidence synthesis: Several factors were identified that need to be taken into account to further develop an organ-sparing treatment approach for early stage localized PCa and turn this concept into clinical practice. First, novel thermoablative techniques such as third-generation cryosurgery, high-intensity focused ultrasound (HIFU), vascular photodynamic therapy and, electroporation can precisely target a tumor lesion within the prostate while maintaining the integrity of the surrounding tissues. Second, new ultrasound, magnetic resonance imaging (MRI) and molecular imaging techniques may provide new means to detect small PCa lesions. Third, extended image-guided biopsy protocols using a transperineal rather than a transrectal approach can provide a more exact spatial distribution of PCa lesions within the prostate. Fourth, careful patient selection using an individualized approach is a prerequisite for optimal preoperative planning and a successful treatment outcome. Conclusions: For patients with early stage localized PCa limited to one focus or lobe and who express a great desire not to jeopardize their QoL, targeted focal therapy will likely play a more significant role in the future as a tangible treatment option. Moreover, focal therapy may fill the gap between active surveillance for low-risk PCa and radical treatment for higher-risk forms. |
| Focal therapy for prostate cancer: revolution or evolution? | Ryan Turpen and Charles J Rosser* |
The face of prostate cancer has been dramatically changed since the late 1980s when PSA was
introduced as a clinical screening tool. More men are diagnosed with small foci of cancers instead of the advanced disease evident prior to PSA screening. Treatment options for these smaller tumors consist of expectant management, radiation therapy (brachytherapy and external beam radiotherapy) and surgery (cryosurgical ablation and radical prostatectomy). In the highly select patient, cancer specific survival employing any of these treatment options is excellent, however morbidity from these interventions are significant. Thus, the idea of treating only the cancer within the prostate and sparing the non-cancerous tissue in the prostate is quite appealing, yet controversial. Moving forward if we are to embrace the focal treatment of prostate cancer we must: be able to accurately identify index lesions within the prostate, image cancers within the prostate and methodically study the litany of focal therapeutic options available. |
| Patient Selection for Hemiablative Focal Therapy of Prostate Cancer | Thomas J. Polascik, MD1, Janice M. Mayes, BSc1, Florian R. Schroeck, MD1, Leon Sun, MD, PhD1, John F. Madden, MD, PhD2, Judd W. Moul, MD1, and Vladimir Mouraviev, MD, PhD1" |
BACKGROUND: The application of focal therapy for low-risk prostate cancer (PCa) depended on appropriatepatient selection. No definitive criteria existed to characterize patients who may potentially benefitfrom an organ-sparing approach.We evaluated pretreatment clinical parameters that may predict unilateralPCa amenable to hemigland thermoablation.
METHODS: In total, 538 patients with complete data from the Duke Prostate Center (DPC) Outcomes database with low- to low-intermediate–risk PCa (prostate-specificantigen <10 ng/mL, biopsy Gleason score 7, and clinical stage T1c-T2b) treated with radical prostatectomy(RP) were included in the dataset. Patients underwent diagnostic prostate biopsy (PBx) at Duke orcommunity hospitals from 1996 to 2006. Clinical and biopsy parameters were assessed as to the ability to predict PCa unilaterality verified by RP pathology. RESULTS: The strongest predictor of pathologic unilaterality was PBx unilaterality. The sensitivity and specificity for biopsy unilaterality to predict pathologic unilateralitywas 88.4% and 34%, with a positive predictive value of 28% and a negative predictive value of 91%.PBx unilaterality (odds ratio [OR] ¼ 3.88; 95% confidence interval [CI], 2.14-7.05; P < .0005) and negativefamily history of PCa (OR ¼ 1.83; 95% CI, 1.09-3.05; P ¼ .21) was associated with a higher probability of unilateraldisease by multivariate regression. CONCLUSIONS: Two pretreatment clinical variables were significantly predictive of unilateral PCa: negative family history of PCa and PBx unilaterality. These variables maybe used to select men with low- to low-moderate–risk PCa for hemiablation. Further work is necessary todecrease the false-negative and false-positive rates associated with PBx to improve predictability for PCa laterality. |
| 限局性前立腺癌に対する高密度焦点式 超音波療法を用いた局所治療の可能性と 課題 | 小路直J. 2). *中野まゆらは) 朝長哲朗1.2) 金伯士1,2) Nagata Yoshil、iro Terachi Toshiro Uchida Toyoaki 長田恵弘1,2) 寺地敏郎1) 内田豊昭1,2) |
小路直J. 2). *中野まゆらは) 朝長哲朗1.2) 金伯士1,2) Nagata Yoshil、iro Terachi Toshiro Uchida Toyoaki 長田恵弘1,2) 寺地敏郎1) 内田豊昭1,2)
|
| 前立腺癌局所治療 | 小路直(東海大学医学部外科学系泌尿器科学) |
要旨近年, 前立腺癌のうち, 臨床的に予後に影響するとされる癌病巣の局在診断が可能であると考えられるように
なったことで, 局所治療が注目されている. 局所治療は,根治的治療とActive Surveillanceの中間に位置する治療概 念であり, 患者の予後に影響すると考えられる癌病巣を治療する一方, 正常組織を可能な限り温存し癌治療と患者 の機能温存を両立することを目的とするものである. このため, 局所治療が限局性前立腺癌の選択肢に加わること で, それぞれの患者に対して, 従来よりも個別化された治療戦略を立てることが可能になると思われる. しかし, 今 後解決すべき課題もあり, 実施にあたっては, 患者の理解を十分に得ることは当然のこと, 厳格な患者選択, 正確な 癌局在診断に基づいた治療計画, 安全で正確な治療方法,そして効果判定方法を示した上で, 治療は行われることが重要である. また, 今後, 局所治療を限局性前立腺癌に対する治療選択肢のーっとするためには, 多くの症例の長期成績を集積する必要がある. |
| HIFUを用いた前立腺癌局所療法の可能性 | 小路 直・日暮太朗・川上正能・中野まゆら・内田豊昭 |
要旨 局所療法は,根治的治療とActive Surveillanceの中間に位置する治療概念と考えられ,患者の予後に影響する
と考えられる癌病巣を治療する一方,正常組織を可能な限り温存し,癌治療と患者の機能温存を両立することを目的 とするものである.高密度焦点式超音波療法(high intensity focused ultrasound,以下HIFU)は,治療領域を自 由な形に設定できること,数ミリ単位で治療領域,非治療 領域の組織変化の違いを鮮明にして治療することができる ことから,前立腺癌に対する局所療法に適した治療法として期待される.しかし,これまでに報告された局所療法の 臨床成績は,その目的の一つである機能温存については,有用性が示されている一方,治療効果判定方法が施設間で異なり,経過観察期間も短期間である研究が多いため,その治療効果の有用性については,明らかではない.現在, われわれは,HIFUを用いた局所療法のプロトコールを作成するため,“前立腺癌の局在診断”,“正確な治療の実 施”,そして“治療効果判定および再発評価”の方法について,基礎的,臨床的研究を行っている.今後,国際的な コンセンサスや,これらの研究結果を考慮して作成されたプロトコールのもとで治療が行われ,さらに多施設共同研 究,他治療とのランダム化比較試験により,HIFUを用いた局所療法の有効性について,評価が行われることが望まれる |
By Dr. 小路 直 東海大学医学部外科学系泌尿器科学
臨床論文 動画視聴
HIFU, which stands for high intensity focused ultrasound, uses high frequency ultrasound energy to create heat at a specific point which destroys tissue in the prostate. Sonablate 500 is powered by the unique T3 Technology, which allows physicians to:1) Target specific tissue using integrated ultrasound imaging and sophisticated planning tools, 2) Treat targeted tissue with pinpoint accuracy while sparing untargeted tissue; and 3) Track procedure results using real-time ultrasound imaging along with advanced tissue change monitoring software.
Mechanism of Action
Sonablate® 500 HIFU Ablation™ Software

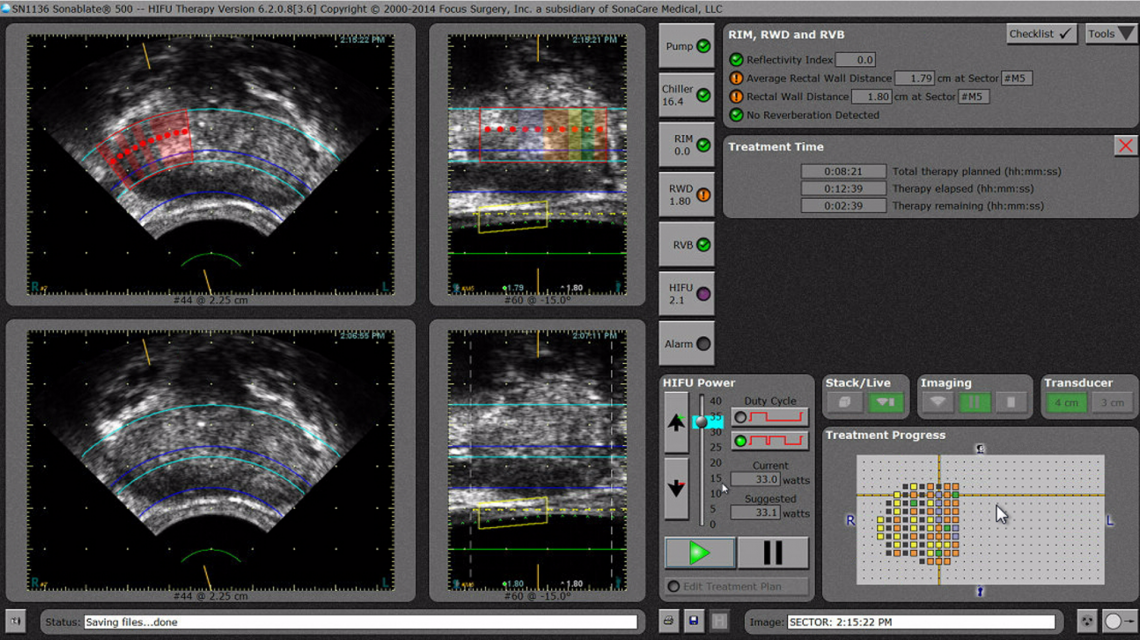
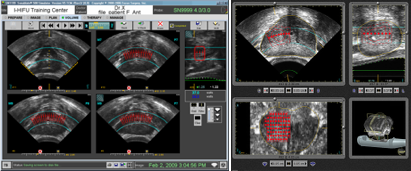
Red shaded areas (upper left) indicate where HIFU has been delivered.
Orange, yellow and green shaded areas (upper right) show how much the tissue has changed. Orange indicates lots of change while green means there was only a small change.

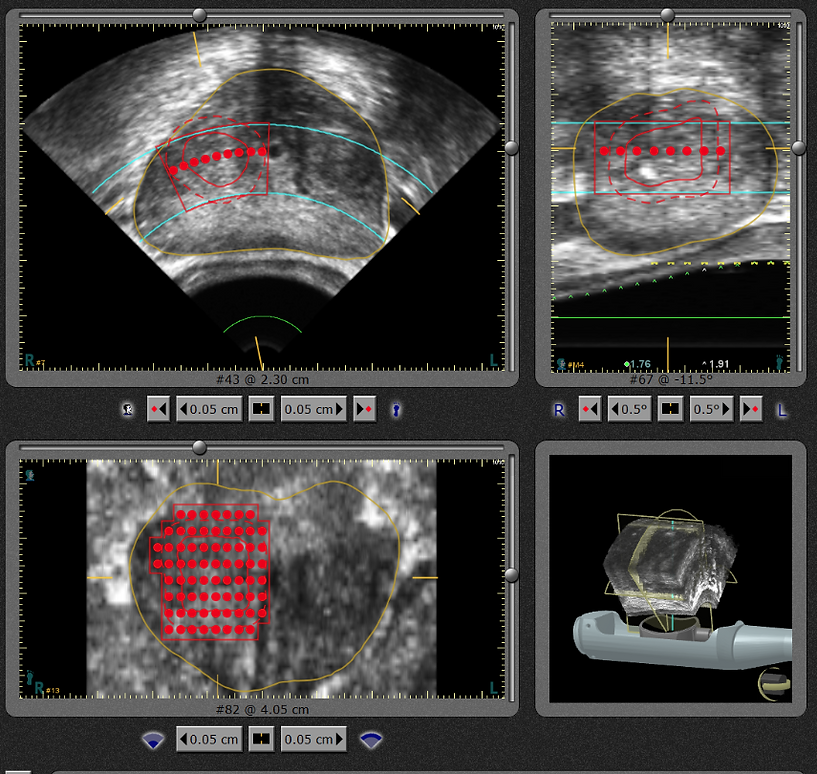
Red dots indicate where doctor has planned to ablate prostate.
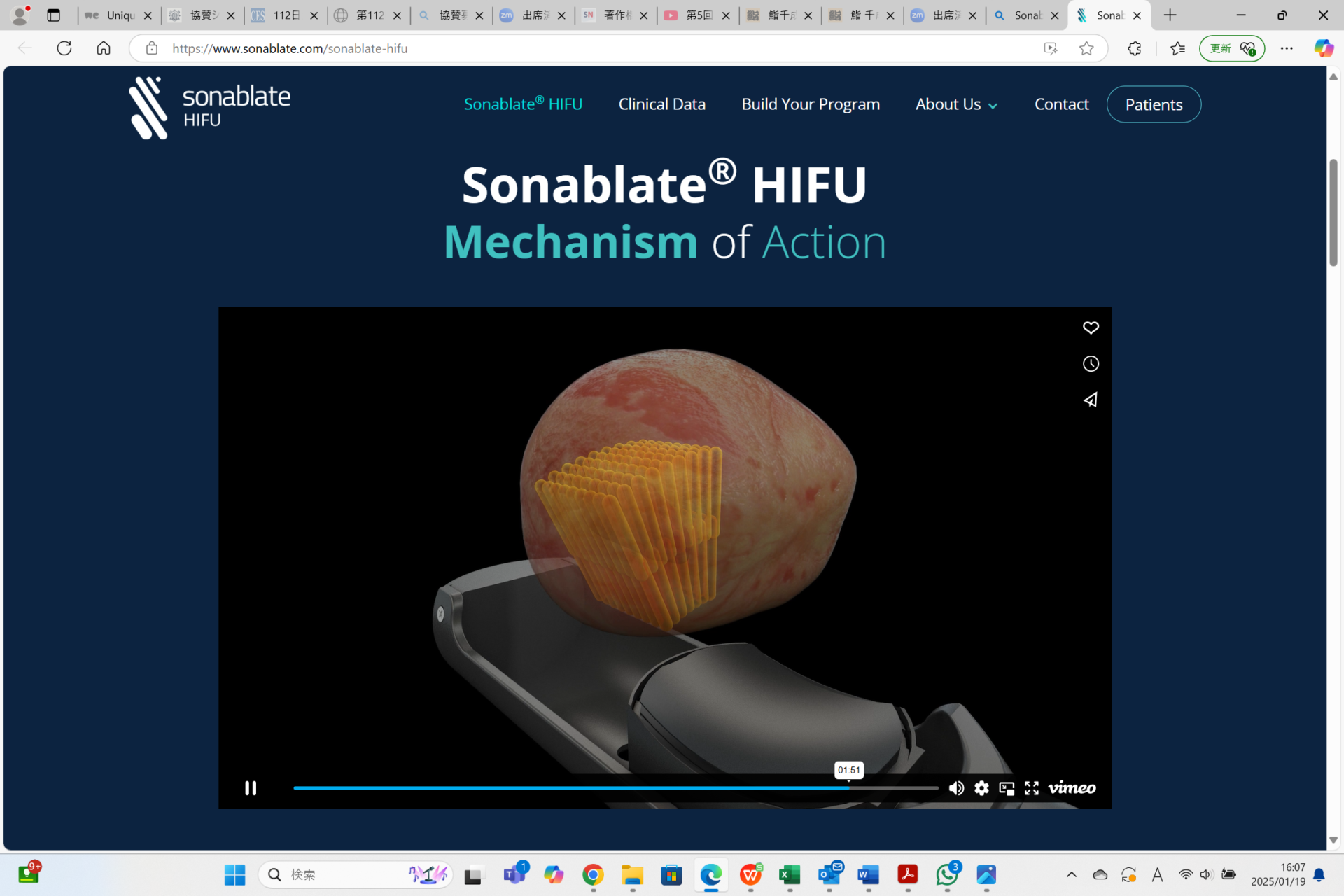
The procedure uses energy-rich bundled ultrasound. The ultrasound energy travels from a transducer which is located in a probe placed in the patient’s rectum.
When HIFU is delivered to a specific location, the corresponding tissue area can be heated up to between 80°-100°C over a maximum period of 3 seconds, resulting in tumour tissue destruction, while the tissue outside the focal point remains unharmed.
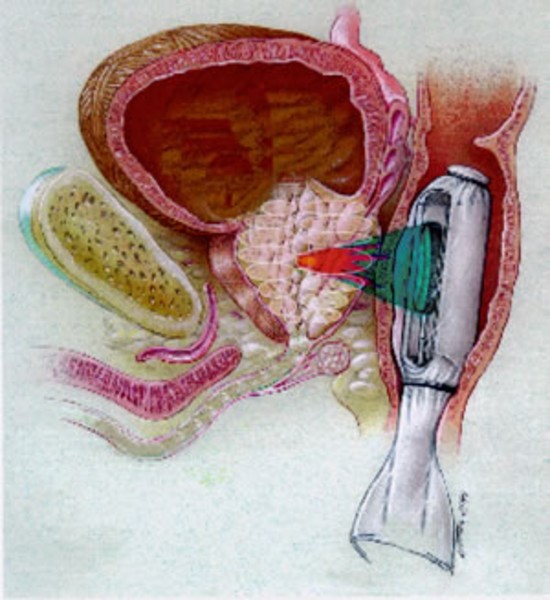
Powerful ultrasonic waves to be irradiated from the inserted probe from the rectum, and then irradiated with only the selected range of the prostatic urethra of through seminal colliculus from the bladder neck.
This treatment is not only less physical burden of patients, it is possible to reduce the cost of treatment from shortness of hospital stay and less needs of necessary consumables of the hospitalization period. Sonablate500 will be able to reconcile the QOL of the patient’s and NEEDS of medical institutions at once.
The Sonablate 500 is integrated with MRI / Ultrasound fusion system with enhanced features to allow for more accurate fusion.
Import data for focal treatment planning from multiple Fusion functionality provided by MIM Software
Import data for focal treatment planning from multiple fusion partners:

Fusion functionality provided by MIM Software
| Transducers | Spherical shell-shaped piezoelectric | |
| Positioning | Ultrasonic tomographic image (linear-sector type) | |
| Treatment monitoring | Ultrasonic tomographic image (linear-sector type) | |
| Power supply | AC100V (± 5%), 50 / 60Hz, 15A, medical 3P ground | |
| probe | Focal length | 30mm, 40mm |
| frequency | 4MHz | |
| Treatment | Treatment unit intensity (SPTA) | 1680W / Cm 2 (30Mm focus)
1300W / Cm 2 (40Mm focus) |
| Irradiation size | 2mm × 2mm × 10mm | |
| Treatment cycle | 4 seconds irradiation, 12 seconds pause (image updates) | |
| Therapeutic range | 0.04Cm 2~ 12Cm 2 | |
| monitor | 15 inches flat LCD monitor | |
| Recording of the image | GIF file (saved to the hard disk) | |
| Printer | Digital graphics printer | |
| Brand Name | Sonablate 500 | |
| Medical equipment approval number | 30100BZX00070000 | |
| Adaptation | Prostatic hyperplasia | |
For the Contents related to the treatment and products, please contact us Takai Medical Industry Co., Ltd.
Or Sonablate Corp for those outside Japan.
Details: Sonablate HIFU | Sonablatecorp
TOP